Ivan Korolev
ESR6
Outotec & Aalto University
My name is Ivan, I am the ESR 6 and I am Russian mining engineer. As many of you may know, my homeland is pretty rich in natural resources, such as oil, diamonds, metal ores. I am myself coming from a small mining town in Siberia, so my choice of profession was of a little surprise.
During the studies in Kuzbass State Technical University, I was focusing mostly on coal mining and processing, as this was the most abundant mineral resource in my region. However, already in the course of my undergraduate studies I have realized that coal use in the world is decreasing from year to year due to raising environmental concerns and strong orientation of the society towards sustainable energy sources.
That is why, soon after receiving my BEng, I decided to make a shift in my area of expertise and fortunately I got the opportunity to continue my education with an Erasmus Mundus Master Course in Georesources Engineering – EMerald. The driving force behind this master program is the concept of geometallurgy, which combines ore geology, mining, metallurgy and recycling. These two years were demanding as much as they were rewarding after all. The challenges I faced, like moving from one country to another every six months, or need to work in a diverse multicultural groups, helped to my personal growth. As for my professional viewpoints, I became confident that people should more carefully treat available natural resources and look for improved technologies of metal recovery and recycling instead of digging more holes in the Earth’s face and turning it into moonscape.
The European countries have inherited a massive legacy from centuries of the mining and metallurgical activities carried on their territories represented as a large quantity of industrial waste. Back then, even the best available technologies did not let to fully extract valuable components, leaving some amount of them in process residues. Under the threat of resource scarcity, these rejects shall be turned into a source of critical metals, if the efficient recovery method is discovered.
My current research is devoted to the development of such process for selective electrowinning of valuable metals from complex solutions. I am delighted to have on my side the leading technology provider for metal industry, Outotec Oy, and enthusiastic result-driven research group of Hydrometallurgy and Corrosion from the Aalto University. This work is indeed just a small piece in bigger canvas of the SOCRATES project, but I strongly believe that together with my 15 colleagues in this project we can offer fresh insights to sustainable use of Earth’s resources.
Should you have further interest in my current research project or just want to have a discussion about current state of mining and metallurgical industry worldwide, feel free to drop a couple of lines to : korolev.ivan@inbox.ru
Problem statement & objectives
Natural resources, especially metals, are gradually being depleted from the Earth’s crust. Therefore, secondary sources such as industrial residues, waste and side-streams could potentially act as a more sustainable supply for critical metals. This approach is at the very heart of the circular economy principles and from that point of view, European countries have “inherited” a large quantity of industrial residues since the early days of industrialization. Emerging interest for extraction of minor quantities of valuable metals from complex impure hydrometallurgical solutions is drawn to electrochemical methods as no additional chemical reagents are required and the recovery can be precisely controlled by applying favourable process conditions.
The primary objective of this project is to find the most energy efficient metal recovery route with the lowest investment costs in such a way that very low concentrations of metals can be recovered from process solutions. The effect of process parameters such as temperature, solution purity, flow rate and electricity applied are optimized to achieve good recovery kinetics and purity.
Methods
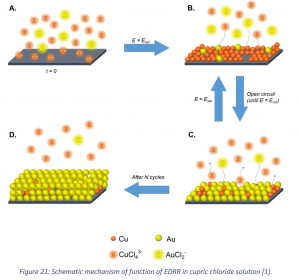
An electrochemical method (electrodeposition-redox replacement; EDRR) has been developed for selective recovery of minor amounts of valuable metals from complex hydrometallurgical solutions. The idea behind the method is that more noble metal (e.g. Au) in the solution would spontaneously react with less noble one (such as Cu) deposited on the solid electrode and hence it will be recovered at the cathode surface (Figure 21). By applying the potential to the electrochemical cell in cycles, the kinetics of gold recovery can be controlled. More details on the experimental setup and conducted analyses of process solutions and gold-containing product can be found in Korelev et al., 2018.
Results obtained during the reporting period
To demonstrate the method in practice, a set of experiments were carried out with synthetic chloride solutions containing Cu and Au in concentrations close to those that could be found in hydrometallurgical solutions; the ratio between metals was Au : Cu = 1 : 340. The objective of the experiments was to obtain electrodeposited product with high concentration of gold and identify the process parameters affecting gold recovery most.
The results of experimental work carried out in Aalto University (Hydrometallurgy and Corrosion Research Group) and Outotec Research Centre (Pori, Finland) indicated the possibility to apply the EDRR method for selective recovery of gold from the hydrometallurgical solutions. Composition of the obtained gold-containing product was Au : Cu = 3.3 : 1, i.e. more than 1000-fold increase in comparison to the gold concentration in the initial solution. In addition, solution analysis showed that 8.4% of Au was recovered from the solution, whereas for Cu the recovery was as low as 0.01%.
In addition, the energy consumption and current efficiency of the EDRR process were compared to the best industrial practices. While the energy consumption is at about the same level for EDRR and conventional gold electrowinning processes, the current efficiency with the EDRR method is much higher – ca. 40%, as opposed to 0.5-1% in conventional gold electrowinning.
After the EDRR was shown to operate in synthetic solutions, batch experiments with actual process solutions from gold ore leaching were carried out. The purpose was to optimize the parameters of the EDRR process for better recovery of gold from the solution. These experiments proved that high purity gold can be recovered with from EDRR method from real process solutions containing gold at ppm scale while copper concentration was as high as 30 g/L and with other impurities (mainly Fe, Zn, Co and Ni) present as well (Korelev et al. 2019). Although the efficiency achieved in the batch tests was ca. 25-30%, it is expected to increase in continuous operating mode.
The next step was to build an experimental set up that would allow for demonstration of the combined electro-hydrometallurgical process for gold traces recovery from flotation tailings on pilot scale. However, this task appeared to be rather challenging from the materials performance perspective due to harsh conditions used in the tests (i.e. high temperatures, acidic solutions, abrasive solids). A key factor for the successful upscaling of the process is a choice of appropriate materials for the equipment that can withstand the aggressive environments. Corrosion tests were performed in order to identify suitable materials for the reactors and electrochemical cells. It was found that commercially pure titanium (Grade 2) is applicable as a material for leaching reactors and moving parts, such as impellers and mixers, whereas a highly-alloyed stainless steel (654 SMO) is a right material for replaceable electrodes in electrolytical cells. The practical implementation of the continuous pilot test, which includes leaching of gold from low-grade raw material followed by its recovery from the solution via EDRR method and final purification of the spent electrolyte is expected to be performed in coming months at Outotec Research Centre’s pilot plant facilities.
Conclusion & Outlook for further work
The results obtained by far are a clear proof not only of the viability of proposed method, but also of its capability to recover gold from solutions at very high selectivity. Quantitative evaluation of the achieved process efficiency and its economic feasibility will be performed following the completion of pilot trials.