My name is Ioanna Maria Pateli, or Marianna, or just Ioanna. Confusing? Yes, it is confusing. What is not confusing to me is that I purely love chemistry, but we will come to that later on.
This September, I graduated from the National and Technical University of Athens and now I am holding a Diploma of Mining and Metallurgical engineer. My specialization is on Metallurgy, which is a very interesting, adventurous and yet dangerous path to walk on. Metallurgy is a whole world of techniques, methodologies, thoughts and, if you recall, it started to develop in 2000 bc, it is undoubtedly clear that without this science, world would be very different. I learned a lot of things, part of which is that chemistry is all around us and has a great impact on everything.
When people were asking me what I want to do after my studies, I was always answering that I wanted to improve the world. How is this possible? Be a researcher is the answer.
I always wanted to be a researcher and you could find evidence in my bedroom’s drawer, which was always full of bottles of homemade chemical stuff. So, when the opportunity knocked on my door, I applied for a PhD position at the University of Leicester. My research topic is “Ionometallurgical leaching using Deep Eutectic Solvents”. Deep eutectic solvents are new, innovative and promising solvents which will change the scenery of Metallurgy in the years to come. My main aim is to dissolve low metal containing industrial residues in ethaline or reline (Deep eutectic solvents) and study the electrochemical behaviour of the metals in these systems. Then, I will try to recover the metals from the solution. Recycling was always a priority of my daily life, so I am really excited to be focused on this topic in my research project. Finally, I am really proud to work with Professor Andrew Abbott, which as I think is one of the most talented professors in his field.
When I first came to UK, I was really afraid top find a cold atmosphere (because of the weather would affect people also) but I was totally wrong. People here welcomed me in the most heart-warming way and I am so grateful for that. The university is very well organised, with many research achievements but the most important thing is that… is located nearby a big green park. Why is this important? Because when you are tired, sleepy after 9 or 10 hours in the lab, you need fresh air and open space to clear your thoughts. So, the thing that I most enjoy here is walking in the park, thinking about my next moves or what to cook for dinner (a wide variety of thoughts in my head).
The most essential thing, in conclusion, is that you have to follow your dreams, wherever they will lead you. Don’t be afraid of the changes in your life, you will always find yourself surprised of how skillful you are. If you are interested in my research project and want to get more information, don’t hesitate to contact me at: patmarianna@gmail.com
Problem Statement and Objectives
The project focuses on the investigation of the dissolution of the metal oxides (MOs) MnO2, MnO, Fe2O3, Fe3O4, Co3O4, CoO, NiO, CuO, Cu2O, ZnO, and PbO in different deep eutectic solvents (DESs). The choice of these MOs was taken due to their existence in many process residues and end-of-life products (EoL) as for example lithium ion batteries (LIBs). In addition, the majority of these oxides can be a primary source of metals. For instance, the iron oxides hematite Fe2O3 and magnetite Fe3O4 along with pyrolusite MnO2 are the main minerals of iron and manganese in the earth’s crust, while in the same time they exist in many industrial residues.
In this investigation, solvometallurgy was employed as an alternative to the state-of-the art methods of processing MOs, which are pyrometallurgy and hydrometallurgy. Both pyrometallurgy and hydrometallurgy are well studied and established in industrial flowsheets, but they suffer from their high environmental footprints. Solvometallurgy, one the other side, is an emerging technology according to Binnemans et al (2017), because it takes advantage of non-aqueous solvents, like molecular solvents, ionic liquids (ILs) and DESs whose tuneable character may result in the development of selective processes for metal recycling.
As mentioned before, in this project DESs are employed, which were introduced in 2003 by Abbott et al. They are eutectic mixtures of quaternary ammonium salts (e.g. choline chloride) with a hydrogen bond donor (HBD). DESs are considered to be a greener alternative when compared to ILs, due to their easier and cheaper preparation by mixing two readily available components, their biodegrability, non-flammability, low vapour pressure and low toxicity. Previous research from Abbott et al (2009) has shown that by using the DES choline chloride-urea, Pb and Zn could be recovered with very high selectivity over Fe from electric arc furnace dust, so they also show potential for the development of selective extraction processes.
Methods
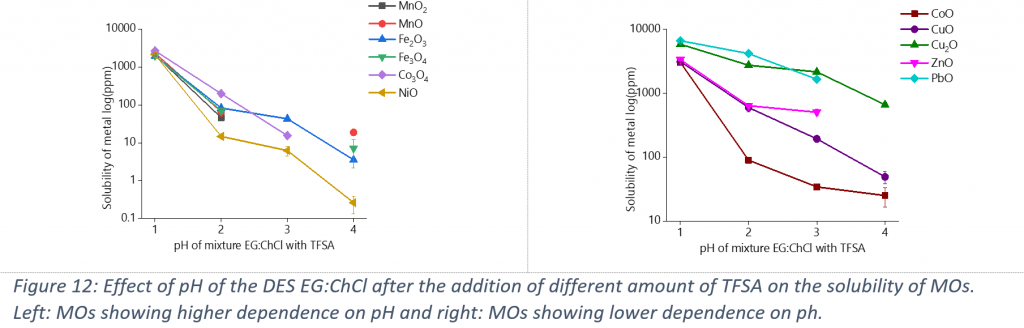
The dissolution of MOs was investigated in different DESs composed of choline chloride mixed with various HBDs (oxalic acid, lactic acid, acetic acid, levulinic acid, ethylene glycol, glycerol and urea). Different parameters can influence the affinity of MOs to dissolve in DESs, like the proton activity of the solvent or the complexing properties of the HBD. In order to understand which factor has the strongest influence and which system can obtain selectivity, the solubility of MOs was tested in different systems. The influence of pH was tested by preparing a series of solutions of choline chloride-ethylene glycol mixed with 10-1, 10-2, 10-3 and 10-4 M of trifluoromethanesulfonic acid. The effect of the complexing ability of the HBD was determined by employing mixtures of choline chloride with various HBDs, other acidic and strong ligands like oxalic acid or neutral molecules like ethylene glycol.
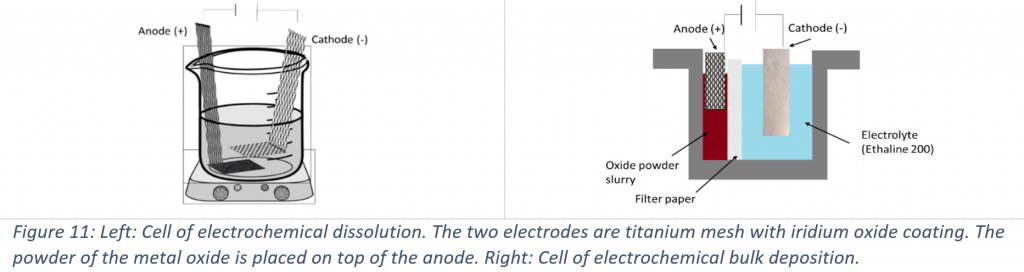
As a second part, the electrochemical dissolution of MOs was investigated in choline chloride-ethylene glycol, in order to examine if by applying voltage to the system the equilibrium of dissolution, in a neutral and not complexing solvent, will be shifted. The dissolution took place for 48h at 50oC with a constant current of 10mA. In addition, the bulk electrochemical deposition of metal oxide slurries was tested in the same DES for 24h at 50oC with a constant current of 2mA and with the cathode to be a nickel plate. For this part, a 3D printed two compartment cell was employed. Both cells are shown in Figure 11.
Fayalite slag was also dissolved in choline chloride-ethaline and in hybrid DESs as choline chloride-ethylene glycol-oxalic acid. The incorporation of metals like Cu, Pb, Zn and Fe in this slag renders it not economically feasible to be processed with DESs, as their price is very low. So, different cases of real EoL products were tested, namely lamp phosphor wastes and spent cathodes of LIBs that include rare earth elements (REEs) and Co, Ni and Mn, respectively.
Results obtained during the reporting period
As is observed in Figure 12, the dissolution for all MOs is higher in the DES with the lower pH, so the proton activity indeed plays a significant role especially for the high oxidation state of MOs. This fact is logical, since protons act as good oxygen acceptors and the breakage of the bond between the metal with oxygen becomes easier.
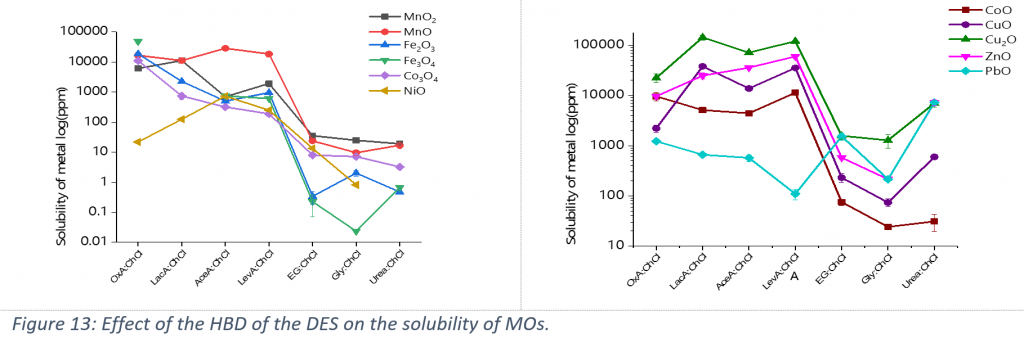
Next, dissolution of MOs in DESs with different HBDs was examined. As is obvious from Figure 13, MOs behave differently and show a more complicated pattern depending on the ligand abilities of the HBD. A very interesting result obtained is the very low solubility of NiO compared with the high solubilities of Co3O4 and MnO2 in choline chloride-oxalic acid, which shows that this DES is a good candidate for recycling of Co and Mn from spent LIBs.
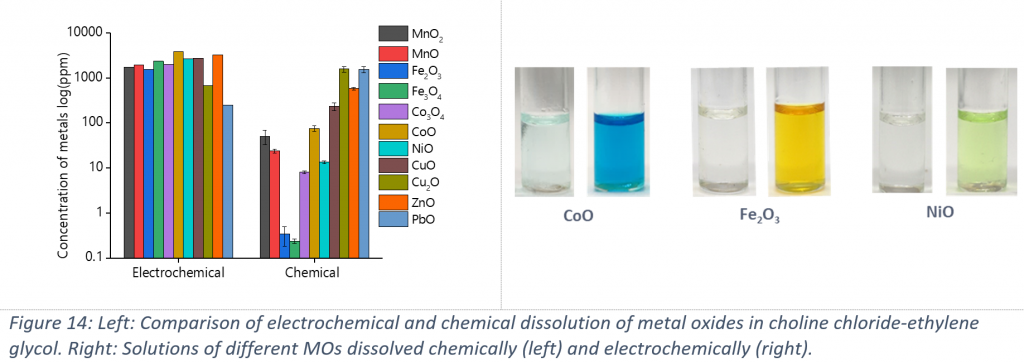
The electrochemical dissolution of choline chloride-ethylene glycol is shown in Figure 14. It can be seen that a neutral solvent with no strong ligand properties can actually dissolve stable MOs electrochemically. It is the first time also that the anodic oxidation of a MO is proven to work in ionic media. It is assumed that by applying positive current, electrons are being removed from the oxygen and the formation of more soluble superoxide anions is the reason of the higher dissolution rates of the electrochemical method.
Fayalite slag dissolution was tested in DESs and especially in the hybrid DES of choline chloride-ethylene glycol and oxalic acid. It was observed that the higher amount of oxalic acid resulted in higher extraction of all metals, but this project was not continued due to the low amount and thus economic value of the metals inside the fayalite slag.
Moving forward, two cases of dissolution of EoL products were examined in DESs. First the recycling of rare earth elements (REEs) from spent fluorescent lamps was tested. Fluorescent lamps are considered as an alternative source of REEs. During the secondment in KU Leuven a solvometallurgical approach for the recovery of REEs from fluorescent lamps was developed. The REEs exist in the phosphor fraction of the lamps and actually, there is a variety of phosphors that can be incorporated in a lamp with the most valuable to be YOX, an oxide of Y and Eu (Y2O3:Eu3+). HALO phosphor (Sr, Ca)10(PO4)(Cl, F)2:Sb3+,Mn2+) also exists but does not contain any REE and is a contaminant. The DES choline chloride-levulinic acid showed high solubility of the YOX phosphor and low solubility of the HALO phosphor, which again proved the selectivity of solvometallurgy. When the DES was compared to pure levulinic acid, very similar leaching behaviour was observed, showing that the proton activity or the ligand properties of the levulinic acid are more important than the chloride. Dissolution of synthetic and real phosphor waste was also investigated in both solvents. Full extraction of Y and Eu was obtained with low co-dissolution of the HALO phosphor in levulinic acid, so the use of choline chloride was not justified. The recovery of the REEs was accomplished by solvent extraction with the acidic extractant bis(2-ethylhexyl)phosphoric acid (D2EHPA) and stripped by an aqueous hydrochloric acid solution. This case was actually very valuable, because it showed that even though DESs show very good potential, sometimes their employment is not required as the individual components behave as well as the DES.
Secondly, the selectivity of the DES choline chloride-oxalic acid towards Co and Mn over Ni, as mentioned before, was validated on a synthetic NMC (LiNi0.33Mn0.33Co0.33¬¬O2) powder that is widely used as active material for the cathodes of LIBs. The recycling of metals from LIBs is a very important topic since their increasing demand will result also in vast amounts of spent batteries and hereby also a potential new source for rare metals like Co. The DES choline chloride-oxalic acid indeed resulted in high extraction of Co and Mn and very low extraction of Ni in the first hour of dissolution, which is a very important result and renders this DES as an alternative to the conventional ways of recycling metals from LIBs.
Conclusion and further outlook
The dissolution of metal oxides was proven to be both influenced by the pH and the complexing ability of the DES, with the latter parameter being more crucial. In addition, selectivity towards specific metal oxides was accomplished by changing the HBD of the DES. For example, it was observed that choline chloride-oxalic acid is able to dissolve very high amounts of Co3O4, CoO and MnO2 and negligible amounts of NiO. This was also tested on the cathode materials of LIBs that incorporate these MOs and the selectivity still existed.
The anodic oxidation of MOs in a neutral solvent as choline chloride-ethylene glycol, was proven to be successful and to increase the rate of dissolution almost 3 or 4 decimal points for most oxides.
In general, DESs are reported to be promising candidates for the extraction of metals from EoL products, but the price of the metal and its concentration is the limiting factor for the decision of the DES and for actually deciding if solvometallurgy can be economically feasible.